 Home >
Solutions - Microwave Ablation - MWA in Liver
Home >
Solutions - Microwave Ablation - MWA in Liver
Liver cancer is the sixth most commonly diagnosed cancer and the third leading cause of cancer death world- wide in 2020, with approximately 906,000 new cases and 830,000 deaths. Primary liver cancer includes hepatocellular carcinoma (HCC) (comprising 75%-85% of cases) and intrahepatic cholangiocarcinoma (10%-15%), as wel as other rare types [1].
Traditionally, surgical resection is the reference standard for treatment of patients with hepatic malignancy, however, only a small proportion of patients have the chance to be candidates [2].
As an minimal invasive therapy, image guided Microwave Ablation technology has been widely used as a clinical treatment option for tumors including liver tumor in China [3] and Asia [4]. Microwave Ablation technology has also been recommended by CSCO Guideline (Level of Evidence 1, Recommendation B) [5] and CIRSE Standard Guideline (Level of Evidence 1) [6] as a first line therapy for solitary hepatocellular carcinoma. NCCN Guideline recommend MWA as a suitable option for selected patients (Recommendation 2A) [7].
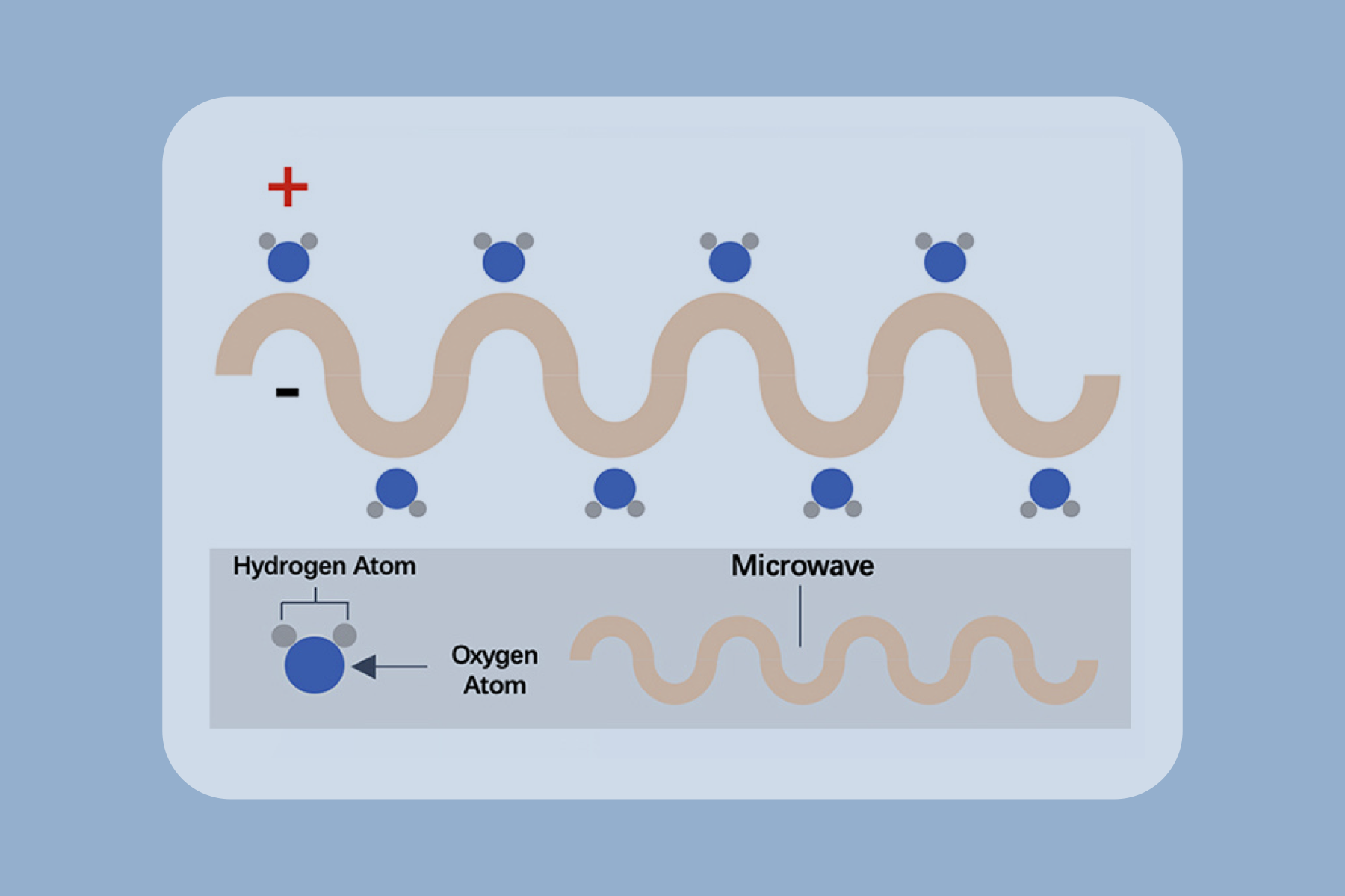
Microwave ablation refers to the use of the electromagnetic methods for inducing tumor destruction by using devices with frequencies of at least 900 MHz to produce tissue-heating effects [4]. Currently, 2450MHz is the frequency used by mainstream products worldwide.
The first clinical study on MWA treating liver cancers was reported by Japan in Seki T. et al.. first performed microwave coagulation in 18 patients with a single unresectable HCC with a diameter of 2cm or less [9]. Since then, several Asian regions have successively employed MWA to treat liver cancers, of which China published most studies. In 2012, A Chinese research group designed the largest multicenter clinical study. Liang P. et al. analyzed the MWA efficacy in treating 1363 lesions of 1007 patients with primary liver tumors. The overall survival rates were 91.2%, 72.5%, and 59.8% at 1 year, 3 years, and 5 years respectively [10].
Another long-term study from January 2008 to October 2019, involving 3385 patients admitted to 12 tertiary hospitals in China. In the result, the 1-, 3-, and 5-year OS rates were 96.4%, 82.6% and 70.7% for the MWA group vs 96.4%, 81.3% and 66.1% for the laparoscopic liver resection group. It showed that microwave ablation and laparoscopic liver resection have similar efficacy and can be considered as first-line treatment options [11].
In conclusion, Microwave Ablation therapy has undergone years of practice in the ablation of liver tumors, and a large number of cases and studies have shown that the MWA is a promising minimally invasive ablation technique for the treatment of solid tumours.
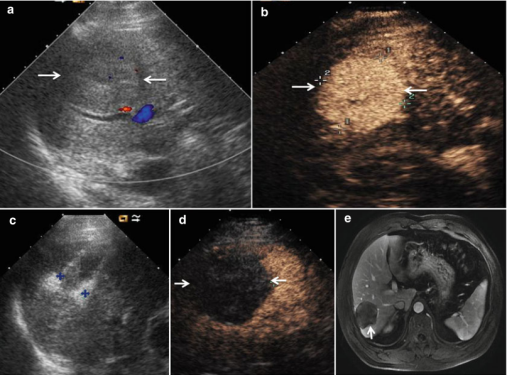
MWA in a 45-year-old woman with HCC on right lobe of the liver. (a) Preablation conventional ultrasound (US) scan shows a hypoechoic lesion with blood supply (arrows). (b) Contrast-enhanced US before MWA shows tumor enhancement in arterial phase with the size of 5.5 × 4.7 cm (arrows). (c) Conventional US shows two microwave antennas (mark) are placed in the tumor. (d) Contrast-enhanced US shows no enhancement of the ablation zone at 12 month after treatment (arrows). (e) MRI scan obtained 21 month after ablation shows hypoat-tenuating ablation zone (arrow) without enhancement [12].
Advantages
Reduced morbidity and mortality

Suitability for real-time imaging guidance

Synergy with other cancer treatments

Less invasive & short hospitalization stay

Repeatability
Cost effectively

1. Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 71(3), 209-249.
2. Liang, P., Yu, J., Lu, M. D., Dong, B. W., Yu, X. L., Zhou, X. D., ... & Lu, G. R. (2013). Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World journal of gastroenterology: WJG, 19(33), 5430.
3. Yu, J., & Liang, P. (2017). Status and advancement of microwave ablation in China. International Journal of Hyperthermia, 33(3), 278-287.
4. Wang, L., Xu, J., Yu, J., & Liang, P. (2021). Review of clinical tumor ablation advance in Asia. International Journal of Hyperthermia, 38(1), 1639-1649.
5. Zhou, J., Sun, H., Wang, Z., Cong, W., Zeng, M., Zhou, W., ... & Fan, J. (2023). Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer, 12(5), 405-444.
6. Crocetti, L., de Baére, T., Pereira, P. L., & Tarantino, F. P. (2020). CIRSE standards of practice on thermal ablation of liver tumours. CardioVascular and Interventional Radiology, 43, 951-962.
7. Benson, A. B., D’Angelica, M. I., Abbott, D. E., Anaya, D. A., Anders, R., Are, C., ... & Darlow, S. D. (2021). Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 19(5), 541-565.
8. Simon, C. J., Dupuy, D. E., & Mayo-Smith, W. W. (2005). Microwave ablation: principles and applications. Radiographics, 25(1), S69-S83.
9. Seki, T., Wakabayashi, M., Nakagawa, T., Itho, T., Shiro, T., Kunieda, K., ... & Inoue, K. (1994). Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer, 74(3), 817-825.
10. Liang, P., Yu, J., Yu, X. L., Wang, X. H., Wei, Q., Yu, S. Y., ... & Han, Z. Y. (2012). Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut, 61(7), 1100-1101.
11. Wang, Z., Liu, M., Zhang, D. Z., Wu, S. S., Hong, Z. X., He, G. B., ... & Liang, P. (2022). Microwave ablation versus laparoscopic resection as first‐line therapy for solitary 3–5‐cm HCC. Hepatology, 76(1), 66-77.
12. Feng, B., Mu, M., & Liang, P. (2015). Microwave Ablation of Adrenal Tumors. Microwave Ablation Treatment of Solid Tumors, 217-226.








